According to researchers from the U.S. Food and Drug Administration (FDA), silicone-gel filled breast implants can rupture frequently without women knowing. Breast implant ruptures were believed to be rare and plastic surgeons have been telling patients that ruptures occur in only 1% to 2% of cases. However, the new FDA study revealed that 69% of 344 women who said their silicone implants were fine were found to have at least one ruptured implant.
In the study, magnetic resonance (MRI) breast imaging was performed on the women, all of whom had had silicone implants placed before 1988. In 21% of the women, silicone gel had leaked outside one or both breasts. The researchers expressed concern with the findings, saying that migrating silicone can be dangerous because it is sometimes impossible to remove.
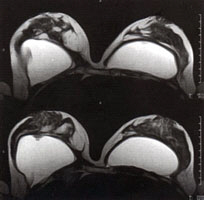 |
Transverse high-resolution MR mammography (MRI) of breast and implants. Note the implant twisting on the upper (left) image and the implant valve on the lower (left) image. |
“In the past, breast implant rupture was thought to be rare, but the FDA study indicates that rupture of silicone gel implants is much more common that previously thought,” said the FDA in a statement. In the study, nearly half of the women with ruptures only had breast implants for six to eight years. The FDA’s investigation of 907 women found that one-third of the women had at least one surgery to have an implant removed or replaced.
Materials scientist Eugene Goldberg, chair of the World Biomaterials Congress Symposium in Hawaii where an FDA official presented the study, said it is uncertain what the long-term consequences of silicone breast implant ruptures are.
In 1992, the general use of silicone breast implants was banned after claims that the implants caused immune-related sicknesses and other neurological disorders. In 1999, a study by the Institute of Medicine found that silicone implants did not cause chronic disorders but implant rupture could pose other concerns, such as infection. Presently, silicone implants may only be used in closely monitored clinical trials .
Approximately one million women in the United States have silicone breast implants. The presentation of this study comes only a few weeks after the FDA announced that saline (salt water) filled breast implants could be kept on the market despite the high rate of complications.
Nearly 135,000 women chose to have breast implant surgery for cosmetic reasons in 1999 while an additional 15,000 breast cancer patients had reconstruction using implants after mastectomy.
- The May 18, 2000 Reuters Health report, “FDA Study Shows High Rate of Breast Implant Ruptures,” is available at http://www.reutershealth.com/archive/2000/05/18/eline/links/20000518elin035.asp
- The May 18, 2000 Reuters Health report, “Silicone Implants Rupture More Than Thought-FDA,” is available at http://dailynews.yahoo.com/h/nm/20000519/sc/health_implants_7.asp
- The May 17, 2000 Imaginis.com report, “FDA Approves Saline Breast Implants Despite Risks,” is available at http://www.imaginis.com/breasthealth/news/news5.17.00.asp
- To learn more about breast implants, please
visit
http://www.imaginis.com/breasthealth/augmentation.asp
- To learn more about breast implant imaging and implant rupture, please visit http://www.imaginis.com/breasthealth/breastimplant1.asp



