The safety and effectiveness of saline-filled (salt-water) breast implants is currently being evaluated by an advisory committee to the U.S. Food and Drug Administration (FDA). Eight years ago the FDA banned the general use of silicone gel filled implants after numerous women reported health problems from manufacturing defects and implant misuse. The FDA did continue to allow the use of saline-filled implants , although saline implants have never been officially approved by the FDA because they went on the market before the FDA began reviewing medical devices. After a 13 hour meeting at which several women with saline implants provided testimony, the FDA advisory committee has recommended approval of at least one brand of saline-filled breast implants. The committee is expected decide whether they will recommend FDA approval of implants made by two other leading manufacturers in the near future.
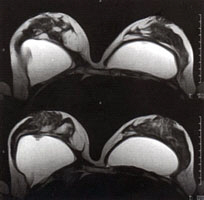 |
Transverse high-resolution MR mammography of breast and silicone implants. Note the implant twisting on the upper (left) image and the implant valve on the lower (left) image |
New attention to saline-filled breast implants comes when the demand for implants is at an all-time high. Nearly 150,000 American women received breast implants in 1999, up from 125,000 women before silicone implants were banned. Implants may be placed in women who wish to enhance the size and shape of their breasts as well as in many breast cancer patients who have had one or both breasts surgically removed ( mastectomy). Approximately 15,000 breast cancer patients chose to have breast implants placed after mastectomy in 1999 while around 135,000 women had implant surgery for cosmetic reasons.
At the FDA advisory committee’s meeting, both positive and negative attitudes toward saline implants were presented. Nearly a dozen women told the committee about physical and emotional problems they experienced from saline-filled implants. Women blamed the implants for infections, severe breast pain , and repeat surgeries. Some women even held up their removed implants, showing the committee how the implants had become blackened with fungus. Physicians note that the most common problem of breast implants is capsular contracture—the scar around the implant begins to tighten and squeezes down on the soft implant, causing the breast to feel hard. However, a recent study shows that the likelihood of capsular contracture may be reduced if implants are treated with antibacterial agents during surgery.
The FDA advisory committee also heard testimony from women who were very pleased with their implants. A number of women said breast implants enhanced their self-esteem and sexuality. One breast cancer patient noted that implants helped tremendously with her emotional recovery from mastectomy (breast removal surgery). Dr. Lin Puckett, MD, head of the American Society of Plastic Surgeons, said that approximately 95% of women are satisfied with their implants.
Though members of the FDA advisory committee said the failure rate from saline-filled implants is "alarmingly high," they recommended approval of Mentor Corporation’s implants because there is no medical evidence that the implants cause any major diseases. However, the committee said that patients must be appropriately warned of the risks involved before undergoing implant surgery. Only one committee member, Nancy Dubler, an expert in bioethics, voted against approving saline implants. She cited the high deflation and leakage rates as key factors in her decision. The FDA is expected to make an official decision on the general use of saline-filled implants at a later date. The FDA does not usually go against the advisory committee’s recommendations.
In a study conducted by Mentor, a manufacturer of breast implants, 27% of 1,680 women with saline implants had to have them removed or replaced within three years of implantation. In the majority of cases, painful scar tissue, infections, and implant ruptures were the causes of additional surgeries. According to Mentor, the women at most risk of implant complications in the study were breast cancer patients who accounted for 27% of the implant removals; only 8% of the removals were done on women who had implants placed for cosmetic reasons.
The following side effects are possible with breast implants:
- Capsular contracture (hardening of scar tissue around the implant)
- Calcium deposits in the breast tissue around the implant (usually non-cancerous but occasionally have to be surgically removed to assure they do not indicate cancer)
- Infection around the implant
- Hematoma or seroma (blood or fluid trapped in the wound)
- Delay in healing
- Shifting of implant (further surgery may be necessary)
- Temporary or permanent changes in the feeling of the nipple or breast (some women report areas of increased or decreased sensitivity or numbness near the incision)
Breast implants may also deflate or rupture from injury to the breast or through normal wear over time. Saline implants tend to deflate quickly, and surgery is usually done immediately to remove or replace the implant. Approximately 50% of saline implants need some type of modification or replacement after five or 10 years. Breast implants also make mammography more difficult to perform and may obscure breast abnormalities from detection on a mammogram. For this reason, several special mammography views are usually taken.
Breast implant surgery is only one option available to breast cancer patients who wish to have the contour of their breast rebuilt after mastectomy. A muscle flap reconstruction procedure rebuilds the breast using the patient’s own tissue. In addition, some women who have their breasts removed choose to wear prostheses (artificial breasts) instead of undergoing surgery. Most prostheses are made to resemble the body’s own weight and touch.
- The February 29, 2000 Reuters Health report, "Safety of U.S. Breast Implants Back in Spotlight," is available at http://news.excite.com/news/r/000229/16/health-implants2
- The March 2, 2000 Associated Press report, "FDA Panel Oks One Brand of Saline Breast Implant," is available at http://www.bergen.com/morenews/breast02200003026.asp
- The March 1, 2000 WebMD report by Ori Twersky, "FDA Advisors Put Breast Implants in Back in the Spotlight," is available at http://my.webmd.com/content/article/1728.55368
- To learn more about breast reconstruction after mastectomy, please visit http://www.imaginis.com/breasthealth/reconstruction.asp
- To learn more about breast implant imaging, please visit http://www.imaginis.com/breasthealth/breastimplant1.asp



